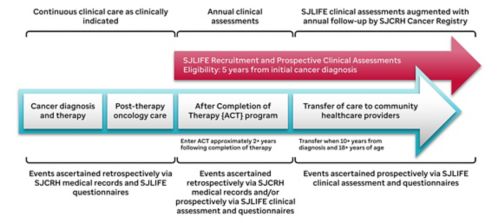
Study Methods
Before the St. Jude LIFE evaluation, survivor medical records are abstracted to ascertain all chemotherapy received, including cumulative doses for 32 specific chemotherapeutic agents, surgical procedures, radiation treatment fields, dose, and energy source, and hematopoietic cell transplantation. Key health events, especially life-threatening organ toxicity, and subsequent malignancies are also obtained and validated by review of medical records. Participants complete a battery of questionnaires that provide patient-reported outcomes of sociodemographics, health status, and interval medical events. The campus evaluation includes a comprehensive clinical assessment including neurocognitive testing and an evaluation of neuromuscular function in the Human Performance Lab, as well as collection of biological specimens. After the campus visit, events reported or newly discovered are followed up by the clinical team and medical records are obtained to validate new diagnoses of health conditions. Specimens in the biorepository are available for future investigations evaluating the association of health outcomes and specific biomarkers and genetic factors.