The St. Jude LIFE study has provided a platform for numerous ancillary and extramurally funded studies. The U01 infrastructure support has facilitated research initiatives addressing a broad spectrum of survivorship outcomes- and intervention-based research as well as an award of a five-year training grant (Krull T32CA225590-01) for post-doctoral Training in Pediatric Cancer Survivorship Outcomes and Interventions, which has a focus on utilization of the SJLIFE cohort.
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
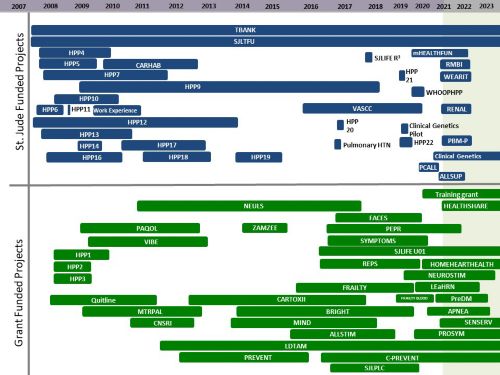
Funded Grants Utilizing SJLIFE Cohort
Efficacy of a Tobacco Quit Line for Childhood Cancer Survivors (QUITLINE)
Principal Investigator: Robert Klesges Funding Source: NIH - 1R01CA127964 Award: $3,801,192 Dates of Funding: 01/29/2008- 11/31/2011
Evaluation of Long-term Effects of Anthracycline Induced Cardiotoxicity
Principal Investigator: Greg Armstrong Funding Source: ASCO Award: $199,998 Dates of Funding: 07/01/2008 - 6/30/2011
Motor Proficiency and Physical Activity in Adult Survivors of Childhood ALL (MTRPAL)
Principal Investigator: Kiri Ness Dates of Funding: 04/01/09 - 02/28/13 Funding Source: NCI - 5R01CA132901 Award: $2,582,369
Risk of Psychopathology and Neurocognitive Impairment in Leukemia Survivors (NEULS)
Principal Investigator: Kevin Krull Dates of Funding: 02/05/2010-12/31/2016 Funding Source: NIH/NIMH (National Institute of Mental Health) 1R01MH085849 Award: $ $2,590,459
Long-Term Treatment Related CNS Injury in Survivors of Childhood ALL (CNSRI)
Principal Investigator: Greg Armstrong Dates of Funding: 07/01/2010 - 06/30/2012 Funding Source: NCI - 1R21CA138988 Award: $484,702
Genome-wide study of late effects of therapy in childhood cancer survivors
Principal Investigator: Carmen Wilson Dates of Funding: 04/01/2011 - 03/31/2012 Funding Source: Rally Foundation Award: $15,000
Genome-Wide Study of Platinum-Based Chemotherapy
Principal Investigator: Carmen Wilson Dates of Funding: 04/01/2012 - 03/31/2013 Funding Source: Rally Foundation Award: $20,000
Low-dose Tamoxifen For Radiation-Induced Breast Cancer Risk Reduction: A Phase IIB Randomized Placebo-Controlled Trial (LDTAM)
Principal Investigator: Melissa Hudson Dates of Funding: 10/01/2011 – 09/30/2017 Funding Source: UAB- NCI - 7R01CA140254 Award: $319,672
Pharmacologic Reversal of Ventricular Remodeling in Childhood Cancer Survivors at Risk for Congestive Heart Failure (PREVENT-CHF): A Phase IIB Randomized Placebo-Controlled Trial (PREVENT)
Principal Investigator: Melissa Hudson Dates of Funding: 10/01/2011- 09/30/2014 Funding Source: COH- LLS Award: $126,660
Longitudinal Cardiotoxicity in Adult Survivors of Childhood Cancer (CARTOXII)
Principal Investigator: Greg Armstrong Dates of Funding: 12/01/2011 - 11/30/2016 Funding Source: NCI - 1R01CA157838 Award: $3,051,065
Melatonin Intervention for Neurocognitive Deficits in the St. Jude Lifetime Cohort (MIND)
Principal Investigator: Tara Brinkman Dates of Evaluation: 07/01/2012 – 05/01/2017 Funding Source: NIH/NCI (Cancer Center Development Funds) P30CA021765 Award: $170,000
Brain Integrity in Survivors of Hodgkin Lymphoma Treated with Thoracic Radiation (BRIGHT)
Principal Investigator: Kevin Krull Funding Source: NCI - 5R01CA174794 Award: $3,912,366 Dates of Funding: 04/01/2013-03/31/2018
A Longitudinal Assessment of Frailty in young adult survivors of childhood cancer (FRAILTY)
Principal Investigator: Kiri Ness Funding Sources: NCI - 1R01CA174851 Award: $3,513,537 Dates of Funding: 05/07/2014 – 04/30/2019
Feasibility and Efficacy of Transcranial Direct Current Stimulation (tDCS) and Cognitive Training for Executive Dysfunction in Adult Survivors of Childhood Acute Lymphoblastic Leukemia (ALLSTIM)
Principal Investigator: Kevin Krull Dates of Funding: 07/01/2014-06/30/2015 Funding Source: NIH/NCI (Cancer Center Development Funds) P30CA021765 Award: $69,000
Impact of resistance training and protein supplementation on lean muscle mass among childhood cancer survivors (REPS)
Principal Investigator: Kiri Ness Funding Sources: AICR Award: $224,560 Dates of Funding: 01/01/2015 – 03/30/2018
The St. Jude Lifetime Cohort (SJLIFE U01)
Principal Investigator: Melissa Hudson/Les Robison Funding Sources: NCI - 1U01CA195547 Award: $8,680,536 Dates of Funding: 07/09/2015 – 6/30/2020
Estimating the Late Effects Disease Burden of Childhood Cancer Survivors
Principal Investigator: Nickhill Bhakta Funding Sources: St. Baldrick’s Foundation Award: $162,881 Dates of Funding: 07/01/2015 – 06/30/2017
Nutritional Status in Adult Survivors of Childhood Cancer
Principal Investigator: Fang Fang Zhang Funding Sources: NCI - 1R03CA199516 Award: $41,008 Dates of Funding: 07/15/2015 – 06/30/2017
Pharmacogenomic of childhood leukemia ALL (SJLPLC)
Principal Investigator: Bill Evans Funding Sources: NCI - 5R01CA036401 Award: $3,651,227 Dates of Funding: 07/25/2015 – 6/30/2020
Accelerating clinical adoption of PROMIS for chronically ill children: The child centered outcomes in practice and research (PEPR)
Principal Investigator: I-Chan Huang Funding Sources: NCI - 1U19AR069525 Award: $88,839 Dates of Funding: 09/30/2015 - 10/01/2019
Symptom progress and adverse health outcomes in adult childhood cancer survivors (SYMPTOMS)
Principal Investigator: I-Chan Huang/Kevin Krull Funding Sources: NCI - 1R21CA202210 Award: $490,438 Dates of Funding: 12/01/2015 - 11/30/2017
Improving cancer screening guidelines for survivors of childhood cancer
Principal Investigator: Jennifer Yeh Funding Sources: ACS Award: $22,374 Dates of Funding: 07/01/2016 - 6/30/2020
Social Cognition and Brain Integrity in Survivors of Pediatric Medulloblastoma (FACES)
Principal Investigator: Tara Brinkman Funding Sources: St. Baldrick’s Foundation Award: $212,866 Dates of Funding: 07/01/2015 - 6/30/2018
Reducing Risk of Anthracycline-related Heart Failure after Childhood Cancer- ALTE1621 (C-PREVENT)
Principal Investigator: Melissa Hudson Funding Source: NCI – R01CA196854 Award: $200,380 Dates of Funding: 07/01/2015 - 6/30/2020
Late effects prediction using clinical phenotypes and whole genome sequencing
Principal Investigator: Yutaka Yasui/Jinghui Zhang Funding Agency: NCI - 1R01CA216354 Award: $3,457,455 Dates of Funding: 04/01/2017 – 03/30/2022
Sleep apnea in survivors of childhood cancer treated with thoracic radiation
Principal Investigator: Kevin Krull/Belinda Mandrell Funding Agency: NCI - 1R01CA215405 Award: $3,757,455 Dates of Funding: 07/01/2017 – 06/30/2022
Training in Pediatric Cancer Care Survivorship
Principal Investigator: Kevin Krull Funding Agency: NIH/NCI, 1T32CA225590-01 Award: $1,149,057 Dates of Funding: 04/01/2018 – 03/31/2023
Social Cognition and Brain Integrity in Survivors of Pediatric Medulloblastoma
Principal Investigator: Tara Brinkman Funding Sources: St. Baldrick’s Foundation Award: $229,998 Dates of Funding: 07/01/2018 - 6/30/2020
A Longitudinal Assessment of frailty in young adult survivors of childhood cancer administrative Supplement
Principal Investigator: Kiri Ness Funding Agency: NCI Award: $ 150,000 Dates of Funding: 09/01/18 – 04/30/20
The St. Jude Lifetime Cohort Administrative Supplement
Principal Investigator: Melissa Hudson Funding Agency: NCI Award: $ 149,972 Dates of Funding: 10/01/18 – 09/30/19
Neurostimulation in Adult Survivors of Childhood Leukemia
Principal Investigator: Tara Brinkman Funding Sources: NIH/NCI, 1R01CA239630-01 Award: $3,275,347 Dates of Funding: 05/01/19 – 04/30/23
Patient-Reported Outcomes Version of the CTCAE for Childhood Cancer Survivors
Principal Investigator: I-Chan Huang Funding Agency: NIH/NCI, 1R01CA218397-01 Award: $3,275,347 Dates of Funding: 08/15/19 – 07/31/24
Prediabetes and Prevention of Diabetes in Survivors of Childhood Cancer
Principal Investigator: Stephanie Dixon Funding Agency: St. Baldrick’s Foundation Award: $169,303 Dates of Funding: 07/01/20 – 06/30/22
Dietary Patterns and Cardiovascular Disease Risk in Childhood Cancer Survivors: SJL Cohort Study
Principal Investigator: Matt Ehrhardt Funding Agency: NIH/WASH U Award: $ 111,190 Dates of Funding: 07/01/20 – 06/30/22
Telehealth based exercise intervention to improve functional capacity in survivors of childhood cancer with significantly limited exercise tolerance
Principal Investigator: Kiri Ness Funding Agency: NIH/NCI 1U01CA246570-01A1 Award: $ 2,101,865 Dates of Funding: 07/01/20 – 06/30/25
Aging-related biomarkers of neurocognitive function in long-term Hodgkin Lymphoma survivors
Principal Investigator: AnnaLynn Williams Funding Agency: NIH/NCI K22/R00 Award: $ 993,773 Dates of Funding: 09/01/20 – 08/30/25
Optimizing stratified cancer survivorship care through multimorbidity risk prediction
Principal Investigator: Melissa Hudson Funding Agency: NIH/NCI SJL Supp Ehrhardt Award: $149,999 Dates of Funding: 07/01/20 – 06/30/21
Personalized dynamic risk stratification model for childhood cancer survivors
Principal Investigator: Melissa Hudson Funding Agency: NIH/NCI SJL Supp Wash U Award: $150,000 Dates of Funding: 07/01/20 – 06/30/21
Epigenetic age acceleration and chronic health conditions among survivors of childhood cancer
Principal Investigator: Melissa Hudson Funding Agency: NIH/NCI SJL Wang Award: $ 66,300 Dates of Funding: 07/01/20 – 06/30/20
Sleep Apnea in Survivors of Childhood Cancer Treated with Thoracic Radiation Supplement
Principal Investigator: Kevin Krull Funding Agency: NIH/NCI Award: $ 448,748 Dates of Funding: 08/01/20 – 07/30/21
Social epigenetic pathways towards health disparities in adult survivors of childhood cancer
Principal Investigator: Zhaoming Wang Funding Agency: V Foundation Award: $599,999 Dates of Funding: 09/01/20 - 08/31/23
Sen-Survivors: An open-label intervention trial for frailty and senescence
Principal Investigator: Greg Armstrong Funding Agency: NIH/NCI, 1U01CA246510 Award: $2,277,711 Dates of Funding: 09/01/20 - 08/31/23
Brain Age in Adult Survivors of Childhood Leukemia and CNS Tumor SJL Supp
Principal Investigator: Melissa Hudson Funding Agency: NIH/NCI-Krull Award: $448,748 Dates of Funding: 07/01/21 -06/30/22
Patient generated health data to predict childhood cancer survivorship outcomes
Principal Investigator: I-Chan Huang Funding Agency: NIH/NCI, 1R01CA258193-01 Award: $ 4,388,082 Dates of Funding: 07/01/21 – 06/30/26
Submitted
Aging Biomarkers accelerated aging and aging-related outcomes among survivors of childhood cancer
Principal Investigator: Zhaoming Wang Funding Agency: NIH
Left Ventricular Non-Compaction in Survivors of Childhood Cancer
Principal Investigator: Dan Mulrooney Funding Agency: NIH 1R21CA253010-01A1
Racial/ethnic differences in risk for frailty among survivors of childhood cancer
Principal Investigator: Carmen Wilson/Kiri Ness Funding Agency: NCI/NIH
An integrated investigation of cardiometabolic risk in childhood cancer survivors
Principal Investigator: Zhaoming Wang/Kiri Ness Funding Agency: NCI
Early identification of childhood cancer survivors at high risk for late onset cardiomyopathy
Principal Investigator: Melissa Hudson Funding Agency: NIH/Loyola-Akbilgi MPI
